What Word Describes the Process of Dissolving
If you cannot see it any more it has dissolved but if you can still see it it has not dissolved. D adding more solid to the liquid.
The relative magnitudes of the energy changes.
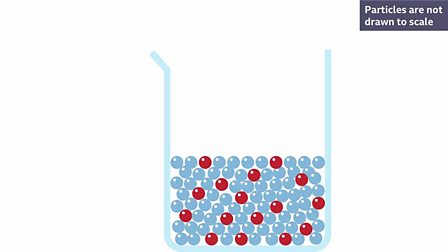
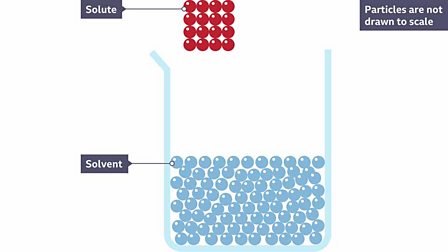
. The process of cations and anions of an ionic solute separating when the solute dissolves. What is the ability of on substance to dissolve in another substance is. So for example in a saline solution salt is the solid dissolved in water where water is the solvent.
This process is referred to as dissociation. The relative magnitudes of the energy changes. This is the process through which a solute makes a solution by breaking the solvent.
Dɪˈzɑːlv dissolve verb BE ABSORBED C2 I or T of a solid to be absorbed by a liquid especially when mixed or of a liquid to absorb a solid. Key Takeaway When a solute dissolves its individual particles are surrounded by solvent molecules and are separated from each other. From Hesss law we know that we can add the energies of each step in the cycle to determine the energy of the overall process.
Solvation is the process of reorganizing solvent and solute molecules into solvation complexes. Does the dissolving is called the solvent. Select all that apply.
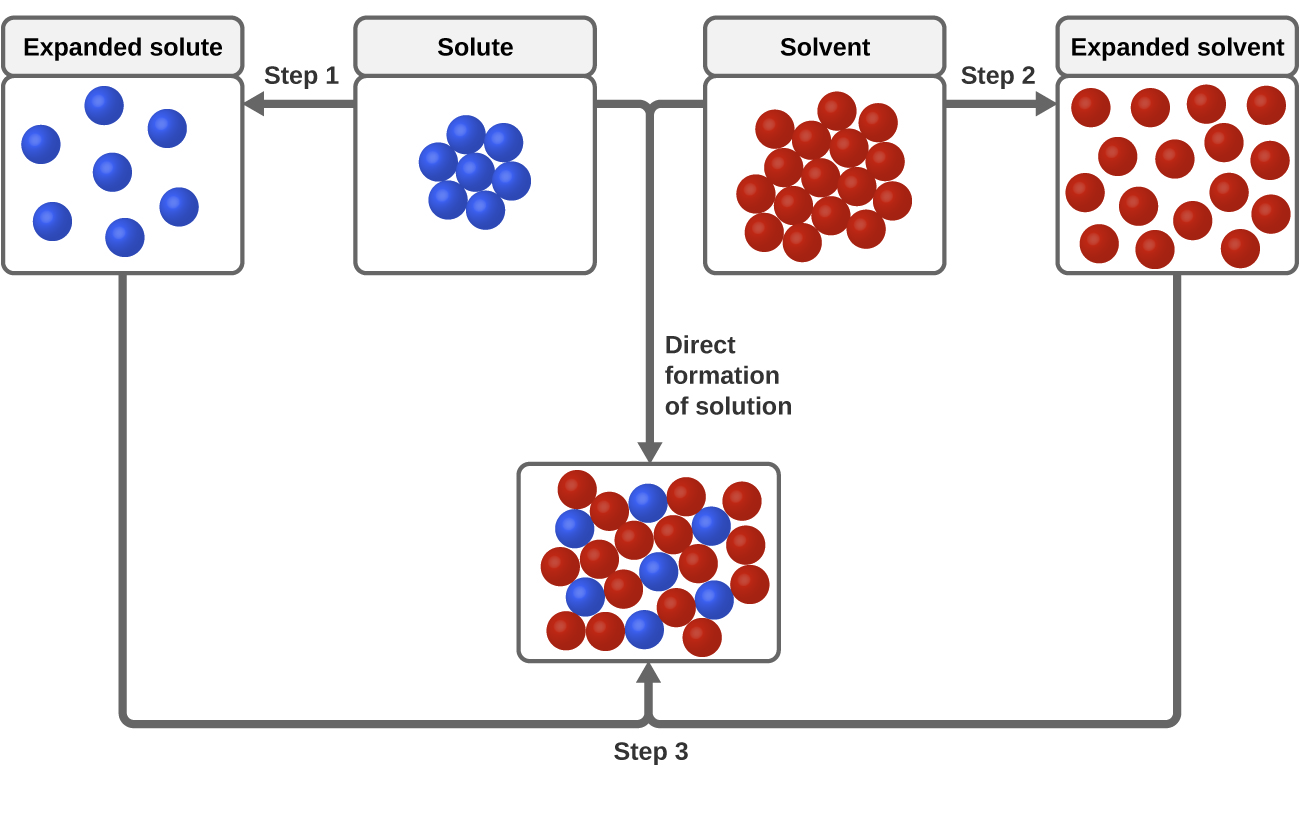
Which is an example of a nonaqueous solution. Compare the dissociation of a simple ionic solute as shown in Figure 95 Ionic Dissociation to the process illustrated in Figure 94 Solvation. As illustrated in Figure 3 the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions endothermic processes and released when solute-solvent attractions are established an exothermic process referred to as solvation.
Give each student a copy of Solubility. B grinding the solid into smaller pieces. The process of dissolving by breaking into smaller pieces is called dissolution.
The process of dissolving is a process which involves the breaking and making of bonds and that involves energy. The concept of dissolving is when a solid called a solute completely mixes with a liquid called a solvent at the molecular level to become a single visible phase and become a. The student determined the density of the sugar-water solution and recorded the collected data in a table.
The sugar in this investigation is. Write a chemical equation that describes the dissolving of solid magnesium nitrate MgNO32 in water. Carry out their own investigations.
Mixture Two or more substances that are mixed together but are not chemically joined. Example the black color in the water soluble felt pen is made up of many colors. Write a chemical equation that describes the dissolving of solid magnesium nitrate MgNO32 in water.
C stirring much more slowly. As illustrated in Figure 3 the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions endothermic processes and released when solute-solvent attractions are established an exothermic process referred to as solvation. Solvation involves bond formation hydrogen bonding and van der Waals forces.
So here were looking at the idea of hydration and the process of dissolving. Dissolving implies that the solute and solvent interact to. Include states of matter in your answer.
Explain how the solvation process describes the dissolution of a solute in a solvent. The ability of one substance to. The solvent is the item that does the dissolving and the solute is the item that is dissolved.
Include states of matter in your. Which of the following would increase the dissolution rate of most solids in a liquid. Dissolving The mixing of a solid with a liquid to make a solution.
It means when you have some water then you add a substance and then stir. What word describes a solid that dissolves in a liquid. When a student has finished check their answers.
Dissolving involves a solute and a solvent. Whats the difference between solvation and dissolution. Which best explains why water has a much higher boiling point than would otherwise be.
Water molecules surround solute molecules. Answer Each particle of the solute is surrounded by particles of the solvent carrying the solute from its original phase. Supervise the students as they.
They can be separated by water because they dissolve in water in different ways. Which statement accurately describes part of the dissolving process of a polar solute in water. Dissolution means the process of dissolving or forming a solution.
A decreasing the temperature of the solvent. Dissolve two spoons of powder in. B grinding the solid into smaller pieces.
And the amount of solid that can be dissolved in a solvent is known as the solid ability. 1 to cease to be visible as the mist dissolved in the morning sun Synonyms for dissolve dematerialize disappear evanesce evaporate fade flee fly melt sink vanish Words Related to dissolve blank out clear die away or down or out disperse dissipate dry up blur dim Phrases Synonymous with dissolve drop out of sight. So the attraction of irons and water molecules.
The density of 200 mL of water was determined and then 20 grams of sugar was dissolved in the water. Student demonstration and the sheet Mass and dissolving. Soluble A substance that can dissolve in a solvent.
Click in the answer box to open the symbol palette. Saturated A solution containing the maximum amount of solute that it can hold. Ask the students to work individually and try to complete the questions.

11 1 The Dissolution Process Chemistry

Condensation Changes In Matter Changes In Matter Condensation Matter Worksheets

Dissolving And Back Again American Chemical Society

Editable One Page Guide Can Be Used As A Review Or Assessment Define Chemical Change Chemical Propert Chemical And Physical Changes Chemical Changes Chemical

Animal Reproduction Worksheet Worksheets Ngss Science Reproduction

Particle Theory Changes Of State

Solute Solvent Solution For Kids Kindergarten Toddlers Preschoolers Solutions Science Videos Science
Solubility Science How Much Is Too Much Scientific American

Halloween Activities Descriptive Writing For Halloween Descriptive Writing Descriptive Writing Activities Writing Activities

Dissolving Process Chemistry For Non Majors

Which Solids Dissolve In Water Cool Science For Kids

Skittles Dissolving Experiment Skittles Experiment Rainbow Experiment Middle School Science Experiments

Pin On Possible Thesis Project
What Are Two Methods By Which A Solid Sample Can Be Made To Dissolve More Readily In A Liquid Solvent Quora